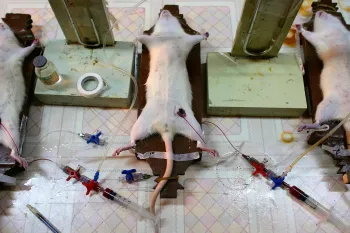
Nearly 1 million horseshoe crabs are taken from the wild every year and used for their blue blood, which contains a special protein that can test for contamination of medical products. Once captured, they are transported to laboratories, where they are strapped into restraints. They are pierced through the heart so up to one-third of their blood can slowly be drained before they are returned to the ocean. After this ordeal, these animals are often too weak to fend for themselves in the wild; many are left dead or injured.
Horseshoe crabs are a keystone species, meaning that other species depend on them for their existence. Over the last few months, the U.S. Atlantic Coast has been home to one of nature’s most remarkable events: the spawning of horseshoe crabs. These ancient arthropods emerge from the depths of the ocean and come ashore to lay their eggs in the sand. Shorebirds, such as the threatened red knot, rely on horseshoe crab eggs as a critical food source as they make their spring migration from South America to the Arctic. These nutrient-rich eggs also provide sustenance for endangered sea turtles and other marine animals.
Horseshoe crabs have existed for almost 450 million years, surviving five mass extinctions. And yet such strength may prove no match for the harm human beings are doing to them, unless we all commit to take action on their behalf.
Scientists have developed effective, non-animal versions of the protein in horseshoe crab blood. These methods, called recombinant technologies, can perform the same tests without compromising the safety of medical products. Recombinant technologies have been around for decades, and pharmaceutical regulators around the world are increasingly giving companies the green light to use them instead of outdated horseshoe crab blood methods.
The transition to these new methods remains slow. Infrastructure changes and associated financial costs also create a perceived barrier for some companies looking to implement recombinant technology; it can take anywhere between two to five years for a pharmaceutical company to replace horseshoe crab blood methods with more sustainable methods.
That’s why we are working to build global momentum for the adoption of recombinant technologies as replacements for this obsolete test. We work collaboratively with scientists, regulators and pharmaceutical leaders to raise awareness and overcome barriers to change.
As a member of the Horseshoe Crab Recovery Coalition, we’re urging manufacturers to transition to these non-animal methods, a move that could save hundreds of thousands of horseshoe crabs from harm. Across the U.S. we’re also advocating for state legislation and other policy changes that would protect these animals, including a bill currently being considered in New York that would prohibit harvesting horseshoe crabs for biomedical purposes.
We’re making progress on this issue all over the world, too. With our support, recombinant technologies have similarly been approved in South Korea, but most companies there haven’t started using them yet. We’ve teamed up with local industry partners through the Animal-Free Safety Assessment Collaboration to support their transition. Meanwhile, in India, we’re conducting research to assess the current state of play and determine how to best support more companies and local regulators in making the switch.
Brazil is currently gathering public feedback about formally accepting one method, called recombinant Factor C, to replace horseshoe crab blood in tests. We are working with recombinant technology suppliers, local companies and regulators to ensure that both the recognition and implementation of this method goes smoothly so that the horseshoe crab test is eliminated.
There is no longer any scientific justification to rely on the blood of horseshoe crabs to determine the safety of medical products. Recombinant technologies are a leading example of a high-performing and sustainable way of avoiding unnecessary animal tests. If we know better, we should do better. And that’s exactly what we’re determined to do. But we need your help. Please join the calls to keep horseshoe crabs in our oceans by adding your name to our petition.
Horseshoe crabs deserve to be allowed to live undisturbed in their natural habitat. Help us protect their existence so they may continue to thrive for years to come.